Read This First¶
The Manual provides important procedures and information on how to operate the scanner and configure the IntraoralScan software correctly and safely. Before attempting to operate the product, read the Manual and strictly observe all warnings and cautions. Pay extra attention to the information from Safety information.
Basic Information¶
I. Product name, model
| Classification | Detail |
|---|---|
| Product Name | Intraoral scanner |
| Model | Aoralscan 3 |
II. Name, residence, contact information and after-sales service of the manufacturer
| Classification | Detail |
|---|---|
| Manufacturer name | Shining 3D Tech Co., Ltd. |
| Production Address | No. 1398, Xiangbin Road, Wenyan, Xiaoshan, Hangzhou, Zhejiang, China, 311258 |
III. Contact Information
| Classification | Detail |
|---|---|
| Manufacturer | Shining 3D Tech Co., Ltd. |
| Address | No.1398, Xiangbin Road, Wenyan, Xiaoshan, Hangzhou, Zhejiang, China |
| Website | www.shining3ddental.com |
| Customer Support | dental_support@shining3d.com |
| Shining 3D’s Representative | Lotus NL B.V. |
| Address | Koningin Julianaplein 10, 1e Verd, 2595AA, The Hague, Netherlands. |
| Telephone | +31644168999 |
| peter@lotusnl.com |
IV. Product performance, main structural composition
| Product performance | Detail |
|---|---|
| Appearance and structure | The appearance should be: Smooth, no cracks, no stains, no obvious deformation. Flexible and reliable for operation. |
| Function control and display | ● Function control: After pressing the scanning button, determine whether the front end of the scanner flashes normally. ● Somatosensory function: swing the scanner in four directions, you can operate the scanning interface. ● Display: Under normal working conditions, when the scanner is opened for scanning, the two-dimensional and three-dimensional imaging of the scanned object (such as teeth) can be seen on the display respectively. ● 3D image processing: After the 3D stereo image is generated, the 3D image can be cropped as needed by using the relevant buttons on the top, bottom and right side. |
| Performance | ● Dental scan imaging: The scanner scans the teeth and gingiva to form a 3D digital model. ● Irradiance: Under normal use of the intraoral scanner, the irradiance is not greater than 1mW/cm2 (Refer to IEC 62471:2006 Photo-biological safety of lamps and lamp systems.). ● Accuracy: Under normal conditions, the scanner is used to scan against a standard (e.g., a plaster standard known to be similar in size to a tooth), obtain its three-dimensional stereoscopic data, and measure key dimensions to obtain measured values. ● Heating of the entrance part of the scanner tip: Under normal working conditions, the intraoral scanner should have heating and anti-fogging function when entering the mouth under working condition. |
| Data interface | USB 3.0, data storage format shall include 3D digital model format .stl, .ply and .obj. |
Main Structural Composition
The Scanner consists of Scanner body, scanner tip, USB 3.0 repeater, power adapter, cradle, USB cable, calibrator, software (V1.0), and encryption module. The software carrier is USB flash drive, and the software release version is V1.0. After the power is switched on, ensure that the USB 3.0 repeater and scanner are powered on. When the power is on, the USB 3.0 repeater indicator is green.
Caution
● It is recommended that users copy the software from the USB flash drive to the computer hard disk before installing the driver.
● Use NVIDIA graphics cards to get the best scanning efficiency.
● Do not insert wireless USB network card in the computer. USB wireless network card will cause USB bandwidth occupation, limiting camera performance.
V. Product maintenance and care methods, special storage/transportation conditions, operating conditions.
| Product Care | Detail |
|---|---|
| Maintenance | Use dust cap when you leave the scanner unworking. |
| Storage | ● Do not connect the scanner to power if not used, keep it in dry environment. ● After using scanner tip, use alcohol to wipe and then use autoclave to sterilize it. (121°C, 102.9kPa for 30 minutes; 134°C, 205.8kPa for 4 minutes). Use alcohol to wipe the scanner body. Use dust-proof cloth to wipe the scanning window to ensure the window keeps dry. |
| Operating temperature | 10°C to 40°C relative humidity: 30%–70%。 |
| Storage/transport temperature | -25°C to 60°C relative humidity: 30%–90%. |
| Air pressure | 86kPa–106kPa |
Note
The temperature and humidity and atmospheric pressure conditions for storage/transportation are mentioned on the outer packaging.
VI. Production date and lifecycle
The production date is shown on the product label. Lifecycle: 8 years.
VII. The list of accessories, including accessories, wear and tear replacement cycle and instructions on how to replace.
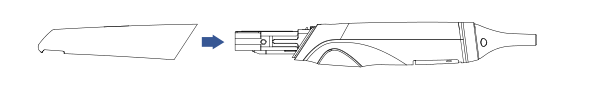
Scanner tip as a wear and tear products can be autoclaved up to 100 times, after which it needs to be replaced.
(1) Disconnect the scanner power, hold the scanner tip firmly with thumb and index finger on both sides, and then gently slide the scanner tip out of the scanner as shown in the figure.

(2) Hold the scanner tip firmly with your thumb and index finger on both sides and gently attach the scanner tip to the scanner with the tip facing down.
Caution
Do not place your fingers on the lens of the scanner tip when removing and attaching the scanner tip, because this might cause damage to the lenses.
(3) Try to gently shake the scanner tip to ensure that it locks into place and is stable.
Caution
● The Aoralscan 3 intraoral scanner should not be used in close proximity or stacked with other equipment, and if it must be used in close proximity or stacked, observe to verify proper operation in the configuration in which it is used. ● Using cables or accessories other than those specified for use with the scanner might result in increased emissions or decreased immunity of the device.
● Interruptions during electrostatic testing can be recovered within 5s without affecting basic performance.
Intended Use¶
This is an intraoral scanner that works with the supplied software programs. By performing intraoral scanning directly and digitally acquiring and saving the 2D/3D color images of teeth and gingiva, the Scanner is available for patients with needs of orthodontic, implant, and restoration.
Note
● l Benefits to be achieved: As a device that applies a probing optical scanner tip, this scanner can directly scan inside the patient's mouth to obtain three-dimensional morphology and color texture information of soft and hard tissue surfaces such as teeth, gums, and mucous membranes in the oral cavity, facilitating comfortable data capturing for patients, reducing stress for medical care, and improving efficiency for following processing.
● The scanner satisfies![]() related requirements.
related requirements.
Caution
● Do not use the scanner for purposes other than those intended and expressly stated above.
● This product is designed and intended for use by persons with professions of dentistry and dental laboratory technology. The product cannot be operated by the patients themselves. The user is solely responsible for determining whether the scanner is appropriate for a particular patient case.
● Do not misuse the scanner, and do not use or operate the software programs incorrectly.
● The clinical environments where the scanner and the software programs can be used include dental clinics, dental hospitals, and dental laboratories.
● Only trained medical personnel may use the scanner and the supplied software programs. When under an adverse event, inform the relevant notified authorities and competent authorities.
● Installation, use, and operation of the scanner are subject to the law in the jurisdictions in which it is used. Install, use, and operate the scanner only in such ways that do not conflict with applicable laws or regulations, which have the force of law. Use of the scanner for purposes other than those intended and expressly stated here, as well as incorrect use or operation, may relieve us or our agents from all or some responsibilities for resultant noncompliance, damage, or injury.
● The users of this scanner and software are responsible for image quality and diagnosis. They should ensure that the inspection data is being used for the analysis and diagnosis only, and furthermore the data is sufficient both spatially and temporally for the measurement approach being used.
● The images acquired by the scanner must be interpreted by a qualified medical professional. The software in no way interprets these images or provides a medical diagnosis of the patient being examined.
Contraindications¶
No known contraindications (or side effects).
Caution¶
● Do not attempt to disassemble, repair, or modify the scanner and software.
● There are no user serviceable parts inside the scanner. Necessary modifications must be made only by the manufacturer or its designated agents.
● Do not allow foreign objects (including all types of liquids) to enter the scanner and its cradle. Water, moisture, etc. may cause a short circuit in the electronic components and lead to malfunction.
● If the scanner tip is accidentally dropped to the ground, check to make sure the lens is not loose before using it.
● If the scanner is inadvertently dropped on the ground or impacted, it must be calibrated before use. If there are still accuracy problems or scanning abnormalities after calibration, please consult technical support.
● Do not drop or apply shock/vibration to this scanner and its cradle. Strong impacts may damage the components inside.
● Do not cut, bend, modify, place heavy objects, or step on the cables. Otherwise, the external insulation may be damaged and result in short- circuit or fire.
● To avoid electrical shock, use only supplied power adapter and connect it only to properly grounded wall outlets.
● The device should not be used adjacent to or stacked with other equipment. If adjacent or stacked use is necessary, the device should be observed to verify normal operation in the configuration in which it will be used.
Waste Electrical and Electronic Equipment¶
Disposal of Waste Electrical and Electronic Equipment and by users in private households in the European Union.
This symbol on the product or on the packaging indicates that this cannot be disposed of as household waste. You must dispose of your waste equipment by handling it over to the applicable take-back scheme for the recycling of electrical and electronic equipment and/or battery. For more information about recycling of this equipment, contact your city office, the shop where you purchased the equipment or your household waste disposal service. The recycling of materials will help to conserve natural resources and ensure that it is recycled in a manner that protects human health and environment.

Disposal¶
The scanner must be reprocessed prior to disposal in order to prevent cross-contamination.
All electrical and electronic devices must be disposed of separately from your other household waste in order to promote reuse, recycling and other forms of recovery, to prevent any potential adverse effects of hazardous substances on the environment and human health, and also to reduce the amount of waste in landfill. This includes accessories such as power adapters, power cords, etc. Do safely dispose of the device and its accessories in accordance with applicable laws and regulations.
For specific information on disposal of your device and the packaging, contact your local distributor or service provider.
Warranty¶
The warranty is void if unauthorized personnel perform service or maintenance on the set of Aoralscan 3. To ensure correct product performance and to obtain warranty service, contact technical support.
Safety Information¶
Precautions¶
Failure to observe the instructions or disregard the warnings may result in damages to the product, personal injury, or even death of the user or the patient.
● Do not use the hardware and software for any application until you have read, understood, and known all the safety information, safety procedures, and emergency procedures contained in the chapter. Operating the hardware and software without a proper awareness of safe use could lead to fatal damage to the hardware or permanent data loss.
● Ensure that the connection is performed correctly. See 5.1 Connect the Scanner.
● Use only medical grade devices with the scanner in the medical environment.
● The hardware and software should only be used in a medical facility under the supervision of trained personnel.
● Only authorized service labs should perform maintenance. It is expressly prohibited to open the scanner with tools.
● The hardware and software have been fully adjusted and tested prior to shipment from the factory. Unauthorized modifications will void your warranty.
● If the hardware or software is modified, appropriate inspection and testing must be conducted to ensure continued safe use.
● Check the scanner and components for sharp edges.
● Before use, check the device for damage, loose parts, wear and tear, and other cosmetic problems. In case of such problems, please contact after-sales service.
● During use, always pay attention to abnormal conditions of the scanner and the patient. In case of abnormal conditions, you need to stop using it immediately. Consult technical support staff promptly.
● To ensure the performance and safety of the scanner, use only the original accessories provided with the scanner (or accessories specified by Shining 3D, consult technical support for details) and software.
● Use only supplied accessories and approved software with the scanner in order to achieve the designed performance.
● Do not use a power adapter other than the one supplied with the package.
● Connecting the scanner to an unknown power adapter is very dangerous and may lead to fire or explosion.
● Using cables or accessories other than those specified for use with the scanner might result in increased emissions or decreased immunity of the device.
● The supplied medical grade power adapter should only be connected to a grounded power socket.
● Reasonably arrange communication cables, power lines and other types of cables to prevent users or patients from tripping over the wires. Do not forcibly pull or bend cables of any kind.
● The scanner is not intended for use in environments with high concentrations of flammable liquids, gases, or atmospheric oxygen.
● There is a risk of explosion when the scanner is used around flammable anesthetics.
● Do not connect USB peripherals with an extended USB cable. Extended connection may cause unexpected usage fault.
● Always handle the scanner with care and avoid hitting or scratching the surfaces as it contains fragile components. Dropping the scanner on the floor may cause permanent damage. If you accidentally drop the scanner, you MUST dispose the scanner tip immediately and do not use the same tip again. The mirror in the tip might shatter into small pieces, and using it again poses the highest risk of causing serious injury to the user and patient.
● The scanner might heat up to above the normal body temperature, yet this short- term exposure and contact with small areas will not pose a health or safety hazard to the patient.
● The scanner may interfere with pacemakers and ICDs, and use of the scanner on patients with pacemakers and ICDs is prohibited.
● Never place any objects or load on the scanner and its cradle.
● Do not dispose the scanner as unsorted municipal waste. The scanner must be collected separately and disposed of in accordance with the local laws and regulations. For proper disposal of this scanner, contact your local representative of Shining3D Corporation.
Labels and Symbols¶
On the Scanner¶
Specification for scanner serial number
Serial number AOS3-AAH001K13 represents the 001 product manufactured on October 13, 2018.
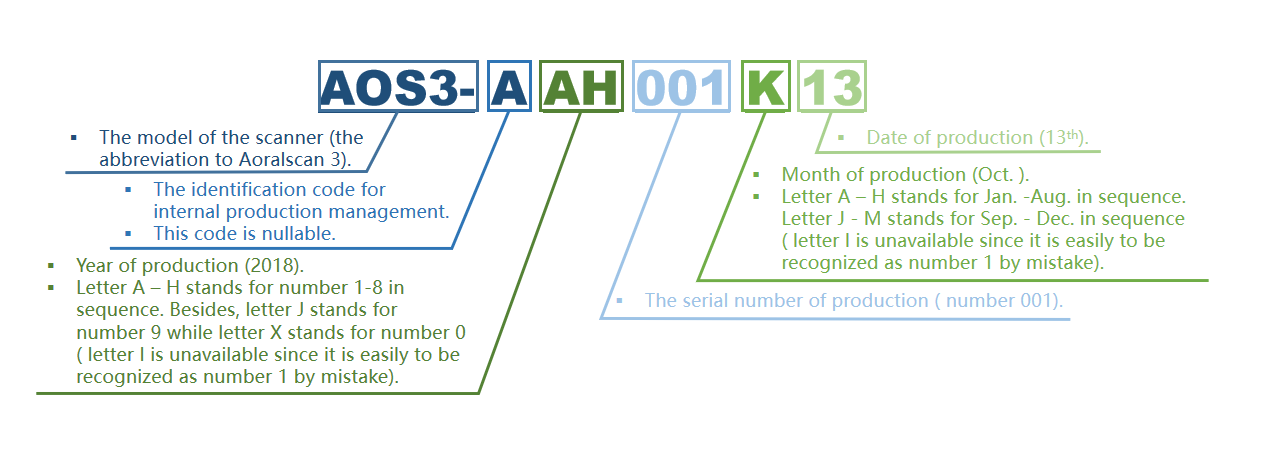
Specification for serial number of calibrator
Serial number iCIII-AH001L05 represents the 001 product produced on November 05, 2018.
● iCIII......Represents the model of the calibrator.
● AH......Represents the year of production, A to J in alphabetical order stands for 1:9 (Since letter I and number 1 are easily confused, ignore I), and 0 is represented by the letter X.
● 001......On behalf of the offline water number.
● L ......On behalf of the month, by letter A-M for 1-12 (Since letter I and number 1 are easily confused, ignore I).
● 05......Represents the date, expressed in 01 - 31.
On the Scanner/Carry Box/Package¶
The following symbols provide information on the product’s labels and regulatory compliance.
| Symbol | Explanation |
|---|---|
| To indicate that caution is necessary when operating the device or control close to where the symbol is placed, or to indicate that the current situation needs operator’s awareness or operator’s action in order to avoid undesirable consequences. | |
 |
Type BF applied part. To identify a type BF applied part complying with IEC 60601-1. |
| Indicate that the contents of the transport package are fragile and the package shall be handled with care. | |
| Indicate that the transport package shall be kept away from rain and in dry conditions. | |
| Indicate correct upright position of the transport package. | |
| Indicate that the marked item or its material is part of a recovery or recycling process. | |
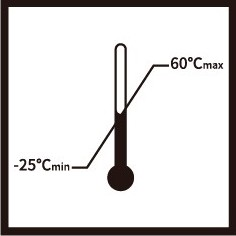 |
Indicate the maximum and minimum temperature limits at which the item shall be stored, transported or used. |
| Indicate the acceptable upper and lower limits of relative humidity for transport and storage. | |
| Indicates the medical device manufacturer. | |
| Indicates the manufacturer's serial number so that a specific medical device can be identified. | |
| Device fulfills the requirements of the European Regulation 2017/745 given on the EU Declaration of Conformity. | |
| Indicate the item is a medical device. | |
| Class II equipment. | |
 |
Class 1 laser product. |
| RoHs | Restriction of Hazardous Substances in Electrical and Electronic Equipment. Meets the requirements of Directive 2011/65/EU. |
 |
Indicates the authorized representative in the European Community/ European Union. |
| Signify that the instruction manual/booklet must be read. |
Note
The symbols meet the requirements of ISO 15223-1 2021"Medical devices - Symbols to be used with information to be supplied by the manufacturer Part1 General requirements".
Compliance¶
Anyone creating or changing a medical electrical system through a combination with other devices in accordance with standard IEC 60601-1:2005+AMD1:2012 Medical electrical equipment – Part 1: General requirements for basic safety and essential performance is responsible for ensuring that the requirements of these standards are met to the full extent in order to ensure the safety of patients, operators and the environment.
FCC Compliance Statement¶
This device complies with part 15 of the FCC Rules. Operation is subject to the following two conditions:
(1) This device may not cause harmful interference,
(2) this device must accept any interference received, including interference that may cause undesired operation.
Electrical Safety¶
Only trained medical personnel should operate this scanner. The product complies with the following standards.
Electrical¶
● IEC 60601-1:2005+AMD1:2012 Medical electrical equipment – Part 1: General requirements for basic safety and essential performance
● IEC 60601-1-2:2014 Medical electrical equipment Part 1-2: General requirements for basic safety and essential performance-Collateral Standard: Electromagnetic disturbances– Requirements and tests
● IEC 60601-1-6:2010+AMD1:2013 Medical electrical equipment – Part 1-6: General requirements for basic safety and essential performance – Collateral standard: Usability
● IEC 60601-1-9:2007+AMD1:2013 Medical electrical equipment–Part 1-9: General requirements for basic safety and essential performance–Collateral Standard: Requirements for environmentally conscious design
● IEC 62366 2007+AMD1:2014 Medical devices–Part 1: Application of usability engineering to medical devices
Classification¶
● Type of protection against electric shock: Class II
● The degree of protection against electric shock: Type BF
● Enclosure protection: IPX0
● Degree of protection against incoming liquids: Common device
● The mode of operation: Continuous operation
● Pollution degree 2
● Level of safety when used with flammable anesthetic gas mixed with air or flammable anesthetic gas mixed with oxygen or nitrous oxide: Non-AP/APG equipment
Caution
The supplier should provide circuit diagrams, component list, figure legends, correction rules and other information needed for technical support staff to repair the parts produced by the manufacturer.
Caution
● Shock hazards exist if the power adapter is damaged or is not properly grounded. Use only the supplied medical grade power adapter.
● To meet waterproof requirements, the sockets should not be placed on the ground.
● Do not use grounding type plugs for other purposes.
● Only authorized service labs can make internal replacements of the scanner and modify the software.
● Do not use the scanner if its tip or cable is damaged. Contact technical support for replacement of the damaged equipment.
● To avoid risk of electrical shock hazards, always inspect the scanner and cable connections before use.
● Check the cable housing before use. Do not use the scanner if the housing is damaged or the cable is abraded.
● All devices connected to the Aoralscan 3 shall comply with IEC 60601-1 and IEC 60950.
● The radiation characteristics of the scanner is suitable for use in all locations ,including domestic and direct connection to the residential public low-voltage supply grid for domestic use.(CISPR 11 Class B).
EMC Notice¶
Caution
● Aoralscan 3 meets the EMC requirements. ● Users should install and use the EMC information provided in the random file.
● The performance of Aoralscan 3 might be affected by a portable or mobile RF communication device. Avoid strong ELECTROMAGNETIC interference when using a scanner, such as near a mobile phone or microwave oven.
● The guidance and manufacturer's statement are shown in the attached table.
● Aoralscan 3 should not be used in proximity to or on top of other devices. If it must be, observe to verify that it works properly in the configuration in which it is used.
● With the exception of cables sold by the manufacturer of Aoralscan 3 as spare parts for internal components, the use of accessories and cables other than those specified may result in an increase in transmission power or a decrease in immunity of Aoralscan 3.
Electromagnetic Emissions
Medical electrical equipment such as the Aoralscan 3 requires special precautions regarding electromagnetic compatibility, and must be installed and put into service according to the following electromagnetic tables.
The Aoralscan 3 is intended for use in the electromagnetic environment specified below. The customer or user of the Aoralscan 3 should assure that it is used in such an environment.
| Guidance and manufacturer’s declaration–electromagnetic emissions |
|---|
| Aoralscan 3 is intended to be used in the following electromagnetic environment. The purchaser or user of Aoralscan 3 should ensure that it is used in this electromagnetic environment: |
| Emission Measurement | Conformity | Electromagnetic Environment - Guidelines |
|---|---|---|
| RF emissions CISPR 11 | Group 1 | The device uses RF energy only for its internal function. Therefore, its RF emissions are very low and are not likely to cause any interference in nearby electronic equipment. |
| RF emissions CISPR 11 | Class B | The Aoralscan 3 is suitable for use in all locations ,including domestic and direct connection to the residential public low-voltage supply grid for domestic use. |
| Harmonic emissions IEC 61000-3-2 | Not applicable | The Aoralscan 3 is suitable for use in all locations ,including domestic and direct connection to the residential public low-voltage supply grid for domestic use. |
| Voltage fluctuations/flicker according to IEC 61000-3-3 | Applicable | The Aoralscan 3 is suitable for use in all locations ,including domestic and direct connection to the residential public low-voltage supply grid for domestic use. |
Interference immunity
The Aoralscan 3 is intended for use in the electromagnetic environment specified below. The customer or user of the Aoralscan 3 should assure that it is used in such an environment.
| Guidance and Manufacturer's Statement - Electromagnetic Interference Immunity |
|---|
| Aoralscan 3 is intended to be used in the following electromagnetic environment. The purchaser or user of Aoralscan 3 should ensure that it is used in this electromagnetic environment: |
| Immunity test | IEC 60601 test levels | Compliance level | Electromagnetic environment–guidance |
|---|---|---|---|
| Electrostatic discharge (ESD) IEC 61000-4-2 |
±8 kV contact ±2,±4,±8,±15 kV air |
±8 kV contact ±2,±4,±8,±15 kV air |
Floors should be wood, concrete or ceramic tile. If floors are covered with synthetic material, a relative humidity of at least 30% is recommended. |
| Electrical fast transient/burst IEC 61000-4-4 |
±2 kV for power supply lines | ±2 kV for power supply lines | Mains power quality should be that of a typical commercial or hospital environment. |
| Surege IEC 61000-4-5 |
±0.5, ±1 kV line(s) to line(s) | ±0.5, ±1kV line(s) to line(s) | Mains power quality should be that of a typical commercial or hospital environment. |
| Voltage dips, short interruptions and voltage variations on power supply input lines IEC 61000-4-11 |
40% UT (100% dip in UT) for 0.5/1 cycle 70% UT (30% dip in UT) for 25/30 cycles 0% UT (100% dip in UT) for 250/300 cycles |
40% UT (100% dip in UT) for 0.5/1 cycle 70% UT (30% dip in UT) for 25/30 cycles 0% UT (100% dip in UT) for 250/300 cycles |
Mains power quality should be that of a typical commercial or hospital environment. If the user of the Aoralscan 3 requires continued operation during power mains interruptions, it is recommended that the Aoralscan 3 be powered from an uninterruptible power supply or a battery. |
| Power frequency (50/60 Hz) magnetic field IEC 61000-4-8 |
30 A/m | 30 A/m | Power frequency magnetic fields should be at levels characteristic of a typical location in a typical commercial or hospital environment. If image distortion occurs, it may be necessary to position the Aoralscan 3 further from sources of power frequency magnetic fields or to install magnetic shielding. The power frequency magnetic field should be measured in the intended installation location to assure that it is sufficiently low. |
| NOTE: UT is the a.c. mains voltage prior to application of the test level. |
| Guidance and Manufacturer's Statement - Electromagnetic Interference Immunity |
|---|
| Aoralscan 3 is intended to be used in the following electromagnetic environment. The purchaser or user of Aoralscan 3 should ensure that it is used in this electromagnetic environment: |
| Immunity test | IEC 60601 test levels | Compliance level | Electromagnetic environment – guidance |
|---|---|---|---|
| Conducted RF | 3 Vrms 150 kHz ~80 MHz outside ISM bands |
3 V (effective value) | Portable and mobile RF communications equipment should be used no closer to any part of the Aoralscan, including cables, than the recommended separation distance calculated from the equation appliance to the frequency of the transmitter. Recommended separation distance: d= |
| EC 61000-4-6 Radiated RF |
6 Vrms ISM bands between 150 kHz and 80 MHz 3 V/m 80 MHz to 2.5 GHz |
6 V (effective value) ISM bands between 150 kHz and 80 MHz 3 V/m |
d= d= Where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer and d is the recommended separation distance in meters (m). Field strengths from fixed RF transmitters, as determined by an electromagnetic site surveya, should be less than the compliance level in each frequency rangeb . Interference may occur in the vicinity of equipment marked with following symbol: |
NOTE 1: At 80 MHz and 800 MHz, the higher frequency range applies.
NOTE 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people.
a Field strength from fixed transmitters, such as base stations for radio (cellular/ cordless) telephones and land mobile radios, amateur radio, AM and FM radio broadcast and TV broadcast cannot be predicted theoretically with accuracy. To assess the electromagnetic environment due to fixed RF transmitters, an electromagnetic site survey should be considered. If the measured field strength in the location in which the Aoralscan 3 is used exceeds the applicable RF compliance level above, the Aoralscan 3 should be observed to verify normal operation. If abnormal performance is observed, additional measures may be necessary, such as reorienting or relocating the Aoralscan 3.
b Over the frequency range 150 kHz to 80 MHz, field strengths should be less than 3 V/m.
c The ISM (industrial, scientific and medical) bands between 150 kHz and 80 MHz are 6.765 MHz to 6.795 MHz; 13.553 MHz to 13.567 MHz; 26.957 MHz to 27.283 MHz; and 40.66 MHz to 40.70 MHz.
To limit exposure to electromagnetic interference from nearby equipment that can degrade image quality or launch warning messages, it is necessary to position the Aoralscan 3 further from sources of electromagnetic interference or install electromagnetic shielding to block unwanted interference. The customer or the user of the Aoralscan 3 should operate the device under EMI conditions that minimize power supply transients, mechanical interactions, vibration, and thermal, optical, and ionizing radiation.
Separation distances
The Aoralscan 3 is intended for use in the electromagnetic environment in which radiated RF disturbances are controlled. The customer or the user of the Aoralscan 3 can help prevent electromagnetic interference by maintaining a minimum distance between portable and mobile RF communications equipment (transmitters) and the Aoralscan 3 as recommended below, according to the maximum output power of the communications equipment.
| Recommended separation distances between portable and mobile RF communications equipment and the Aoralscan 3 |
|---|
| Aoralscan 3 is intended to be used in the following electromagnetic environment. The purchaser or user of Aoralscan 3 should ensure that it is used in this electromagnetic environment: |
| Rated maximum output power of transmitter (W) | Separation distance according to frequency of transmitter (m) | IEC 60601-1-2: 2014 | |
|---|---|---|---|
| 150 kHz~80 MHz d= |
80 MHz~800 MHz d= |
800 MHz~2.5 GHz d= |
|
| 0.01 | 0.12 | 0.12 | 0.23 |
| 0.1 | 0.38 | 0.38 | 0.73 |
| 1 | 1.2 | 1.2 | 2.3 |
| 10 | 3.8 | 3.8 | 7.3 |
| 100 | 12 | 12 | 23 |
For transmitters rated a maximum output power not listed above, the recommended separation distance d in meters (m) can be estimated using the equation applicable to the frequency of the transmitter, where P is the maximum output power rating of the transmitter in watts (W) according to the transmitter manufacturer.
NOTE 1: At 80 MHz and 800 MHz, the separation distance for the higher frequency range applies.
NOTE 2: These guidelines may not apply in all situations. Electromagnetic propagation is affected by absorption and reflection from structures, objects and people.
The medical electrical equipment is suitable for the professional healthcare environment per 60601-1-2:2014. It is suitable for use in physician offices, clinics, hospitals, and other professional healthcare environments except near HF surgical equipment and the RF shielded room of an ME system for magnetic resonance imaging or other environments where the intensity of electromagnetic disturbances is high.
The clinical environments where the device can be used include physician offices, clinics, hospitals, and clinical point-of-care for diagnosis of patients except environments where the intensity of electromagnetic disturbances is high.
Caution
● Using cables or accessories other than those specified for use with the scanner might result in increased emissions or decreased immunity of the device.
● Portable RF communications equipment (including peripherals such as antenna cables and external antennas) should be used no closer than 30 cm (12 inches) to any part of the Aoralscan 3, including cables specified by the manufacturer. Otherwise, it could lead to degradation of the performance of this equipment.
● If immunity test level is higher than those specified in IEC60601-1-2, the minimum separation distance may be lowered. Lower minimum separation distances shall be calculated using the equation specified in IEC60601-1-2 Chapter 8.10.
Biological Safety¶
Meets biological criteria: ISO10993-5: 2009 (Biological evaluation of medical devices — Part 5:Tests for in vitro cytotoxicity); ISO10993-10: 2010 (Biological evaluation of medical devices — Part 10: Tests for irritation and skin sensitization).
Laser Protection¶

This product is a class 1 laser product and is only for maintenance, replacement and removal by professional personnel of the manufacturer or its designated agent (if necessary). If the device is not used, removed or replaced as required, the normal use of the device may be affected and laser radiation may occur. If a laser component is faulty, contact the manufacturer for help.
This product is a class 1 laser product according to "IEC 60825-1:2014 Safety of laser products-Part 1: Equipment classification and requirements", without harmful laser radiation. Users will not be exposed to laser radiation if they operate the equipment correctly according to the instructions.
Users should be aware of optical radiation protection. Bright light is projected from the scanner tip during scanning. As with other light, there may be a temporary reduction in vision or visual residuals. Do not look directly into the light projected by the scanner tip or shine the light into the eyes of others.